Data-Driven Causal Model Discovery and Personalized Prediction in Alzheimer’s Disease
Haoyang Zheng, Jeffrey R Petrella, P Murali Doraiswamy, Guang Lin, Wenrui Hao, Alzheimer’s Disease Neuroimaging Initiative, in npj Digital Medicine, 2022.
Abstract: With the explosive growth of biomarker data in Alzheimer’s disease (AD) clinical trials, numerous mathematical models have been developed to characterize disease-relevant biomarker trajectories over time. While some of these models are purely empiric, others are causal, built upon various hypotheses of AD pathophysiology, a complex and incompletely understood area of research. One of the most challenging problems in computational causal modeling is using a purely data-driven approach to derive the model’s parameters and the mathematical model itself, without any prior hypothesis bias. In this paper, we develop an innovative data-driven modeling approach to build and parameterize a causal model to characterize the trajectories of AD biomarkers. This approach integrates causal model learning, population parameterization, parameter sensitivity analysis, and personalized prediction. By applying this integrated approach to a large multicenter database of AD biomarkers, the Alzheimer’s Disease Neuroimaging Initiative, several causal models for different AD stages are revealed. In addition, personalized models for each subject are calibrated and provide accurate predictions of future cognitive status.
Description:
More than six million American people suffer from Alzheimer disease and the number is still soaring by nearly five hundred thousand every year. Unfortunately, we are still unable to find a viable treatment to cure or prevent it, or even significantly slow its progression. The reason is the incomplete understanding of the complex disease mechanisms, and the treatment may vary at an individual level.
How to better understand the biomechanism in Alzheimer disease? How to develop an operational modeling approach for the personalized treatment of Alzheimer disease? Professor Guang Lin and I, joint with Professors at Duke University and Penn State University, took over one year to develop the first purely data-driven model to analyze biomechanics inherent in Alzheimer disease progression. The work was based on imaging and genetic, blood- and CSF-based, imaging, and cognitive biomarkers in large cohorts of subjects, which were collected by the Alzheimer’s Disease Neuroimaging Initiative (ADNI).
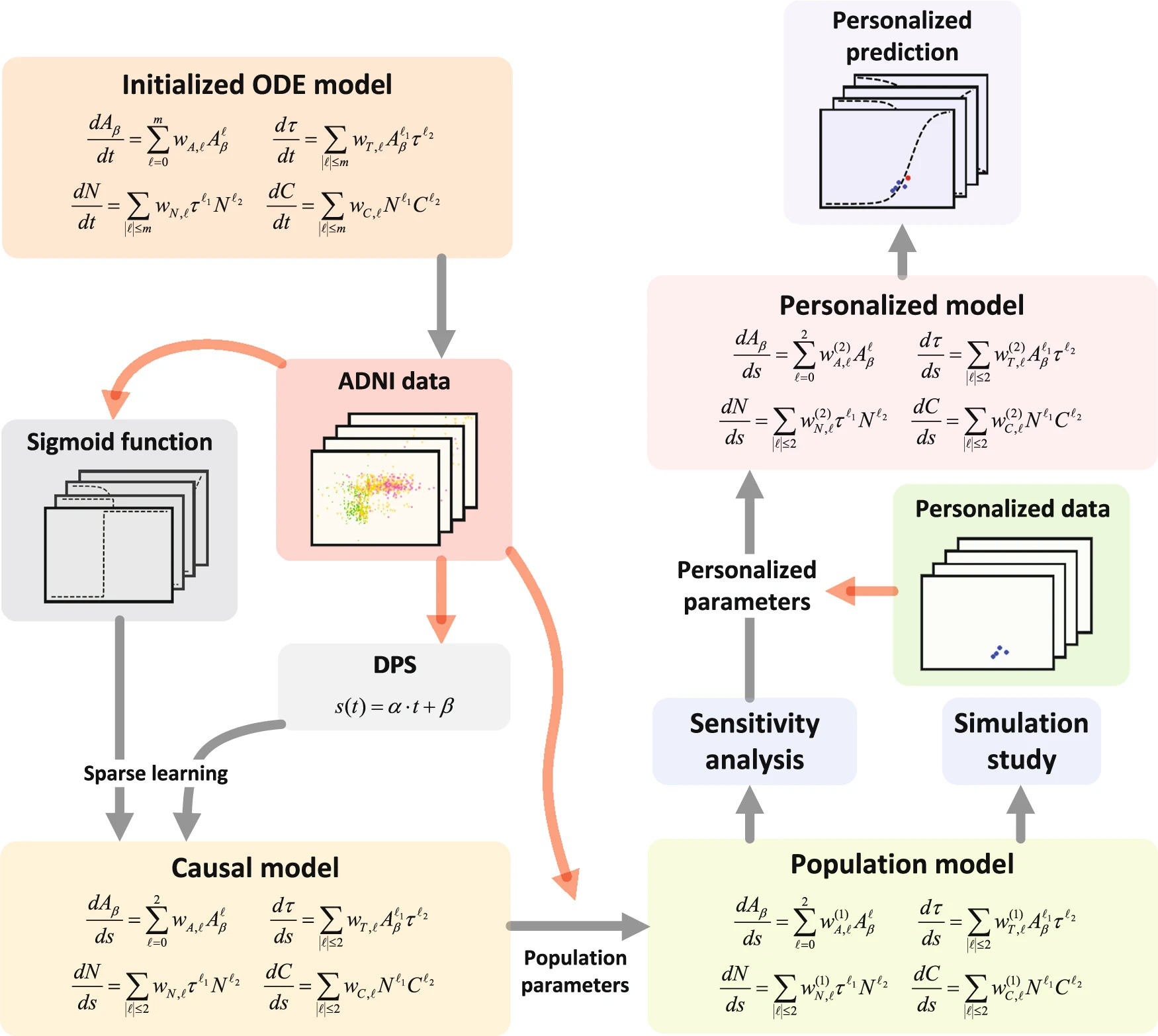
Our work reveals the biomechanics at the group and individual levels and significant pathophysiological pathways in Alzheimer disease. Start by the group level, a population model learned from four biomarkers (amyloid-beta pathology, total-tau pathology, hippocampal volume, and cognitive decline) to describe general Alzheimer disease progression. Based on the population model, we analyzed how biomarkers vary and interact to create a clinically and biologically heterogeneous phenotype and determine the pathophysiological pathways that influence the disease progression most. The determined significant pathways finally resulted in treating patients with varying underlying pathophysiology in a similar fashion and optimizing their treatment planning.
This work was fortunately recognized by two leading Alzheimer researchers, Professor Petrella and Professor Doraiswamy because it advances the understanding of the complexity of Alzheimer disease biomarker pathophysiology over that of current biomarker models which have primarily been independent and ad hoc in nature. We hope the work can benefit Alzheimer disease early diagnosis and personalized predictions.
